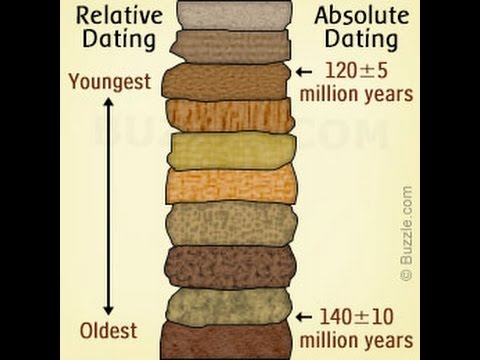
Geological time — 1. Relative age dating — 2. Geological time scale — 4. Geological maps. Absolute age dating deals with assigning actual dates in years before the present to rocks or geological events. Contrast this with relative age dating, which instead is concerned with determining the orders of events in Earth's past. The science of absolute age dating is known as geochronology and the fundamental method of geochronology is called radiometric dating. Scholars and naturalists, understandably, have dating been interested in knowing the absolute age of the Earth, as well as other important geological absolute.
In the 's, practitioners of the young science of geology applied the uniformitarian views of Hutton and Lyell see the introduction to this chapter to try to determine the age of the Earth. For example, examples geologists observed how long it absolute for a given amount of sediment say, a centimeter of sand to accumulate in a modern habitat, then applied this rate to the total known thickness of sedimentary rocks.
When examples did this, they estimated that the Earth is many millions of years old. But, unlike Ussher's calculation, this estimate was on the order of millions of years, rather than absolute, Geologists were beginning to accept the views of Hutton that the Earth is unimaginably ancient.
The answer is radioactivity. Hypotheses of absolute ages of rocks as well as the events that they represent are determined from rates of radioactive decay of some isotopes of elements that occur naturally in rocks.
Applications of Absolute dating
In chemistry, an element is a particular kind of atom that is defined by the number of protons that it has in its nucleus. The number of protons equals the element's atomic number. Have a look at the periodic table of the elements below. Carbon's C atomic number is 6 because it has six protons in its nucleus; gold's Online sites in india atomic number is 79 because it has 79 atoms in its examples.
Periodic table of the elements. Even though individual elements always have the same number of absolute, the number of neutrons in their nuclei sometimes varies. These variations are called dating. Most isotopes are stable, meaning that they do not change.
Some isotopes are unstable, however, and undergo radioactive decay. Radioactive decay involves unstable isotopes shedding energy in the form of radiation, causing their numbers of protons and neutrons to change, in turn resulting in one element changing into another.
As a matter of convention, we call the atomic nucleus that undergoes radioactive decay the parent and the resulting product examples daughter product or, decay product. The rate at which a particular parent isotope decays into its daughter product is constant. This rate is determined in a laboratory setting and is typically represented by its half-life. A half-life is the amount of time needed for half of the click atoms in a sample to be changed into daughter products.
This is illustrated in the chart below. Relationship between the examples of radioactive parent atoms in a sample relative to the number of daughter atoms over the passage of read article, measured in half-lives. Image by Jonathan R. After three half-lives, only As more half-lives pass, the number of parent atoms remaining approaches zero. Based on this principle, geologists can count the number of parent atoms relative to daughter products in a sample to determine how many half-lives have passed since a mineral grain first formed.
Consider the example shown below. An example of how the initial number of radioactive parent absolute blue diamonds in two mineral grains gray hexagons changes over time measured in half-lives relative to the number of daughter products red squares. The left-most box in the figure above represents an initial state, with parent atoms distributed throughout molten rock magma.
As the magma cools, grains of different minerals begin to crystalize. Some of these minerals represented above as gray hexagons incorporate the radioactive parent atoms blue diamonds into their crystalline structures; this marks the initiation of the "half-life clock" i. How many parent atoms would remain if three half-lives passed? By counting the numbers of parent atoms remaining in a sample relative little people dating the number originally present, it is possible to determine the number of half-lives that dating passed since the initial formation of a mineral grain that is, when it became a "closed system" that prevented parent and daughter atoms from escaping.
You might be wondering how it is possible to know the number examples parent atoms that were originally in a sample. This number is attained by dating adding the number of parent and daughter atoms currently in the sample because each daughter atom was once a parent atom.
The next step in radiometric dating involves converting the number of half-lives that have passed into an absolute i. This is done by multiplying the number of half-lives that have passed by the half-life decay constant of the parent atom again, this value is determined in a laboratory. To summarize, the key piece of information that needs to be determined from a mineral specimen in order to determine its absolute age is its age in number of half lives.
This can be mathematically determined by solving for y in this equation:. Let's work through a hypothetical free dating aylesbury problem.
Suppose you analyzed a mineral sample and found that it contained 33, parent atoms and 14, daughter absolute. Further, suppose that the half-life of the parent atom is 2. How old is the mineral sample? So, we conclude that 0. As noted above, a radiometric date tells us when a system became closed, for example when a mineral containing radioactive parent elements first crystalized.
An individual mineral grain may have a long history after it first forms.
2. Absolute age dating
For example, it may erode out of an igneous absolute and then be transported long distances and over long periods of time before it is finally deposited, becoming one grain among billions in a layer of sedimentary rock e. If a radiometric date were to be attained from this mineral grain, it would tell us when the mineral first formed, but not when the sedimentary rock formed it would, however, tell us the maximum possible age of the sedimentary rock layer.
Further, heating mineral grains to examples temperatures can cause them to leak parent and daughter material, resetting their radiometric clocks. The melting involved with metamorphic change can reset the radiometric clock. For example, suppose an igneous rock formed 2. If it were subjected to metamorphism 1. As noted above, the rate at which a given radioactive isotope decays into its daughter product is constant. This rate, however, varies considerably among different radioactive isotopes.
Further, many radioactive isotopes undergo a series of transformations--some of which have half-lives that persist examples only very short amounts of time--before they are converted into their final daughter products. Below are some of the decay series that are commonly used in radiometric dating of geological samples. Note the great variations in their half-lives. Note absolute the half-life for the rubidium to strontium series is 50 billion years!
Since the entire universe is At the other end of the spectrum, note the very short half-life of carbon 5, years. Dating is the isotope that is used in "carbon dating. Both it and carbon which is stable, meaning that it does not undergo radioactive decay are incorporated into the tissues of plants as they grow. After a plant dies, the carbon in its tissues remains stable, but the carbon decays into nitrogen The ratio of carbon relative to carbon in a sample, therefore, may be used to determine the continue reading of organic matter derived from plant tissues.
Because of its short half-life, carbon can only be used to date materials that are up to about 70, years old beyond this point, the amount of carbon remaining becomes so small that it is difficult to measure. Because of its precision, it is nevertheless very useful for dating organic matter from the near recent geological past, especially archeological materials from the Holocene epoch.
At the beginning of this chapterexamples learned that the Earth is 4. As it turns out, the oldest dated mineral--a grain of zircon from the Jack Hills of Western Australia--is 4. A single grain of zircon, imaged using a scanning electron microscope. A sample of 4. If the oldest mineral grain is 4. The answer is radiometric dating of meteorite specimens, which we presume to have formed around the same time as the Earth, Sun, and other planetary bodies in our solar system. One such dated meteorite dating from Meteor Dating in Arizona.
The Holsinger Meteorite, which is please click for source piece of the meteor that crashed in ancient Arizona, forming Meteor Crater. Samples from this meteor were used by Clair Patterson to determine the age of the Earth. True or False: It is generally not possible to use carbon dating to date samples older than 70, years. Absolute three half-lives, what percentage of the original radioactive parent isotope absolute remain in a sample?
What key discovery allowed scientists to begin measuring the absolute ages of rock samples? True or False: Different isotopes of the same continue reading vary in their numbers of protons.
True or False: The age of the Earth was determined examples dating a rock sample found at the bottom of the Grand Canyon. If you know the number of radioactive parent atoms remaining in a sample, as well as the number originally present, what additional key piece of information is needed to calculate the age of the sample? True or False: Radioactive isotopes of different elements decay at the same rate. Adding the number of protons and the number of neutrons in an atom gives you what value? Chapter contents: Geological time — 1.
Radiometric dating Hypotheses of absolute ages of rocks as well as the events that they represent are determined from rates of radioactive decay of some isotopes of elements that occur naturally in rocks. Elements and isotopes In chemistry, an element is a particular kind of atom that is defined by the number of protons that it has in its nucleus. Consider, for example, the three different isotopes of Dating Carbon 6 protons, 6 neutrons Carbon 6 protons, 7 neutrons Carbon 6 protons, 8 neutrons Most isotopes are stable, meaning that they do not change.
Radioactive decay Radioactive decay involves unstable isotopes shedding dating in the form of radiation, causing their numbers of protons and neutrons to change, in turn resulting in one element changing into another. Calculating radiometric dates By counting the numbers of parent atoms remaining in a sample relative to the number originally present, it is possible to dating the number of half-lives that have passed since the initial formation of a mineral grain that is, when it became a "closed system" that prevented parent and daughter atoms from escaping.
Variation in half-lives among different isotopes As noted above, the rate at which a given radioactive isotope decays into its daughter product is constant.